Brave early adopters are the hallmark of an innovation set to create immense human value. In this story, the innovation is cutting-edge technology for interfacing with the nervous system, and the brave early adopter is Ian Burkhart.
Ian, a young man in his mid-twenties, suffered a C5 spinal cord injury (SCI) during a diving accident in 2010. The result was bilateral paralysis below his elbows. With an admirably positive attitude and a wonderfully helpful community, Ian was able to make modifications to his life and still live with a sense of meaning.
One of the primary factors motivating Ian forward through the years of rehabilitation was the strong hope that, at some point in his lifetime, there would be biomedical advances capable of giving him back use of his hands.
As it happened, such a possibility crossed his path.
 Image: https://ianburkhart.com
Image: https://ianburkhart.com
In 2014, Ian was presented with an incredible but uphill opportunity led by Dr. Chad Bouton of Battelle (he’s now with the Feinstein Institute): a team of researchers was looking for a patient to test out a system for restoring movement to the hands of a paralyzed patient. The catch was that it required brain surgery and extensive training.
There are 5.4 million Americans living with some form of paralysis, and Ian is acutely aware of the challenges they face. But optimism is one of his defining characteristics: given the chance to pioneer a technology that could bring massive human value, he was willing to make a sacrifice. And so, in 2014, Ian risked his life by undergoing electable brain surgery, implanting a manmade electrode array into the left motor cortex of his brain.
Over the four years since the operation, and hundreds of hours of tedious and extremely difficult training, Ian has collaborated closely with a team of researchers to improve the brain-machine interface system. Ian can now use the technology, which acts as a “bypass” to his damaged spinal cord, to play Guitar Hero using his right hand.
 Image: PBS
Image: PBS
The body has two information pathways, the vascular system and the nervous system. The typical conception of physiological medical care is that treatments should leverage the body’s vascular system, as in the case of pharmaceuticals; Ian’s system, in contrast, is based on the principle that medical care can be given by interacting with the nervous system. The names of the fields that embody this innovative approach are bioelectronic medicine (BEM) and brain-machine interfaces (BMI).
Brain-machine interfaces are technological systems that read from and/or write to the brain. Bioelectronic medicine systems are technological systems that read from or write to the nerves in your body (the peripheral nervous system). Both of these types of neural interfaces can provide therapeutic value through the insight to target medical interventions at the nervous system rather than just the bloodstream.
At Loup Ventures, we invest in domains of frontier technology that offer strong investment opportunities built on delivering unique human value-add. Bioelectronic medicine and brain-machine interfaces check both boxes with bold strokes. We’re here to explain these technologies and why you should care about them.
Starting with a story
Ian Burkhart was originally approved by the FDA to participate in a year-long trial using a Utah Array electrode implanted in his left motor cortex. When fully hooked up in the laboratory, the signals recorded from the Utah Array, a type of intracortical multielectrode array, are decoded by a neural decoding algorithm and used to control 130 surface electrodes wrapped around his right forearm (which he can’t move on his own). When current is run through the surface electrodes, it causes muscles in Ian’s forearm to contract in particular ways, ultimately controlling his hand movement. In other words, the researchers aim to understand what Ian’s neural activity looks like when he’s thinking about moving his hand. With this understanding, they can use his neural activity to decipher when he wants to move his hand. Then, knowing that he wants to move his hand, a computer program controls his muscles via the electrodes so he can actually move his hand. In essence, they skip right over the spinal cord injury responsible for his paralysis.
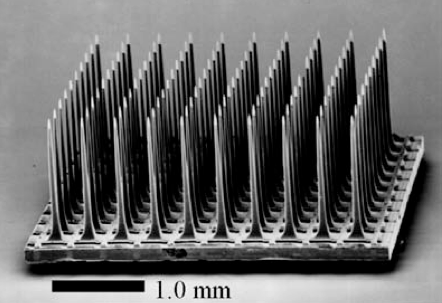 Image: biomedicalcomputationreview.org
Image: biomedicalcomputationreview.org
Ian and his team of researchers have been so successful that the FDA has extended the experiment multiple times; he’s now in his fourth year of participation.
Loup Ventures recently had the pleasure to talk with Ian about his experience and his outlook. Here’s some of what we learned (listen to one of our conversations with him on our Neurotech Podcast here):
- Each day he uses the system, Ian has to retrain the algorithms that decode his neural activity. This is because the specific neural activation representing his thoughts changes frequently due to neural plasticity. When Ian first started with the BMI system, he would leave training sessions mentally exhausted. Over the years, however, it’s become much easier; training isn’t something he has to pay much attention to, now. This has implications for future BEM/BMI technologies: it will be critical to minimize training time and repetition and to make the necessary training as pleasant as possible for the user.
- Ian has spent time learning about the neural decoding algorithms themselves to the extent that he understands the different parameters the research engineers can tune. Now, when Ian is trying to make the BMI perform as well as it can, he can suggest that the researchers adjust specific parameters. This is similar, in a sense, to adjusting the speed of your mouse cursor on your computer screen. In general, this insight from Ian suggests that BEM/BMI systems ought to be designed like any piece of software, where there’s a symbiosis between the user and the machine. The better the user can intuit how the machine will understand him/herself (and vice versa), the more effective the system will be. Ian is very insistent that in order for mass adoption, the technology must be user-friendly; just because its value proposition (i.e. returned movement) is so large doesn’t mean that normal human-centric design considerations can be thrown out the door.
- Even though Ian is very excited about the progress he’s making with the research system in a laboratory setting, he can’t take the system out of the lab yet—partly due to regulation and partly due to the unwieldiness of the technology.
- Even amongst spinal cord injury patients, Ian sees resistance to the adoption of brain-machine interfaces. This paper has great data about which types of interfaces paralysis patients would be comfortable with, given specific types of benefits they can receive. For example, as compared to typing, controlling a cursor, and moving a robotic arm, patients were overwhelmingly most interested in using neural interfaces to control the movement of their own limbs.
- Ian emphasized to us over and over again that brain-machine interfaces and bioelectronic medicine systems must be designed to provide as much value to the user as possible; this bears repetition since most systems are currently in the hands of research scientists and research engineers, not user experience designers.
- We’re discussing a technology that plugs directly into a human brain; this conversation would be woefully incomplete without discussing ethics. Ian pushed this point, since he clearly sees the potential for good in neurotechnology, but acknowledges there’s also potential for bad. One example we think is particularly relevant given current conversation about software platforms is user privacy. User privacy takes on a whole new meaning when the data isn’t just behavioral (like clicking on an ad), but neural. Through the talks he’s given and the other SCI patients he’s spoken with, Ian recognizes that there will be big pushback on technologies that interface with the nervous system, and we think the pushback can be healthy if addressed constructively. With current screen-based interfaces, there have clearly been both positives and negatives. In general, as technology becomes more directly able to “extract” information from, and “input” information into us, the privacy and digital wellbeing stakes get higher. Any foray into the therapeutic benefits of BMI/BEM—whether as an interested onlooker, an investor, a researcher, or a company—would be ill-advised without strong considerations for, and safeguards to protect, the ethical implications of the BMI/BEM product.
In general, we take inspiration from Ian’s conviction about the positive value of nervous system-based interventions, and we take direction from his emphasis on the necessity for BMI/BEM engineers to focus on the practical value of their products. In speaking about his work to improve his own system for movement restoration so that other patients can use it, he says, “I see my involvement with the study as a job that I have to succeed in.” He sees no other choice; the upside is just too large to ignore.
The general bioelectronic medicine approach
Now that we’ve discussed bioelectronic medicine and brain-machine interfaces from the perspective of a real user, we’ll describe these related domains and their benefits to conclude this introductory article. Although the fields of bioelectronic medicine and brain-machine interfaces speak to different use-cases (bioelectronic medicine involves the peripheral nervous system and treats or diagnoses disease in the body; brain-machine interfaces involve the central nervous system and address sensory deficits and cognitive/affective disorders), we’re going to discuss both under the header of “bioelectronic medicine” for the sake of brevity and comprehensibility.
At its highest level, bioelectronic medicine simply uses electronics to interface with the body where previously, chemicals/drugs would have been used. There are three basic components to any BEM system: a body, a device (either inside or outside of the human body), and a computer. In plain English, there are two general ways to use a BEM system:
- A device is used to record electrical or chemical signals from the body, and a computer interprets those signals in order to understand the state of the body or a specific system within it.
- A device is used to stimulate electrical (or other) signals within the body in order to have a desired therapeutic or enhancing outcome, and a computer controls the stimulation.
Some examples of BEM systems include: brain-machine interfaces for restoring movement to paralyzed patients, as in the case of Ian Burkhart; retinal prostheses to return vision to the blind; stimulating the vagus nerve to treat rheumatoid arthritis; and recording electrical signals from the vagus nerve to understand the state of the body’s inflammatory response.
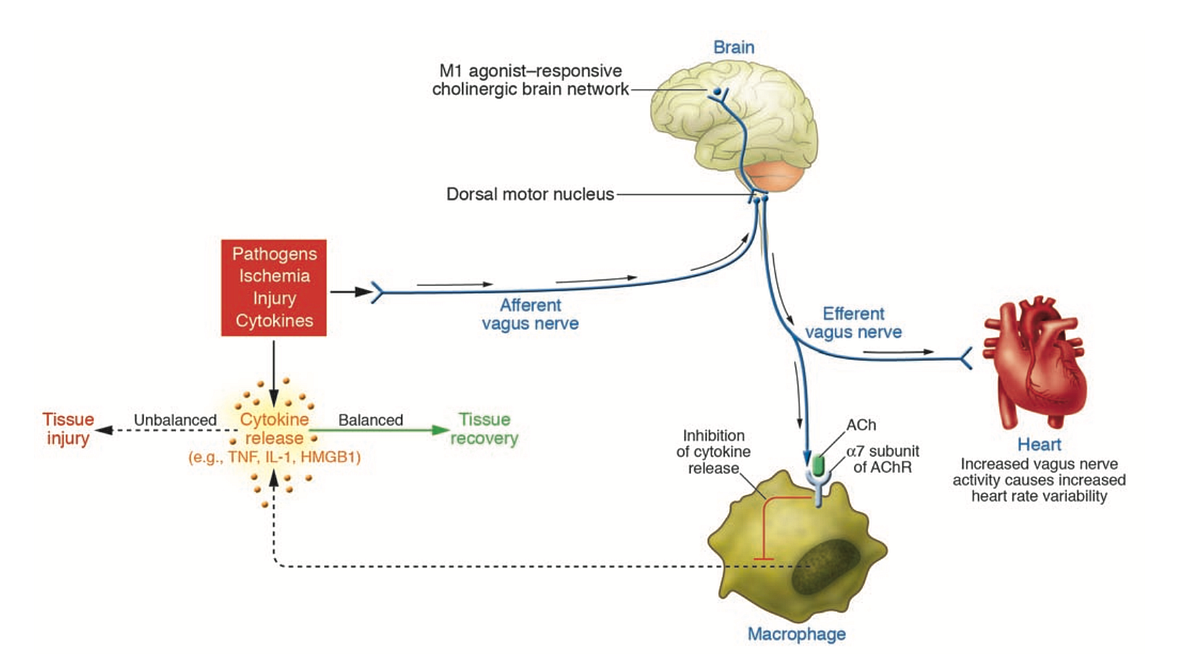 Image: Tracey, 2007
Image: Tracey, 2007
The latter two use-cases exemplify an aspect of bioelectronic medicine that differs from central nervous system-focused brain-machine interfaces: bioelectronic medicine leverages reflexes. In this sense of the word “reflex,” we refer to the idea that the brain both keeps track of and controls organ function using peripheral nerves. This keeping track and controlling is a reflex to preserve homeostasis.
Regulatory and cost advantage
Aside from providing novel treatments and diagnostic opportunities that have yet to be achieved with pharmaceuticals, bioelectronic medicine products offer significant advantages over pharmaceuticals in terms of the FDA regulatory process. All medical devices and drugs are subject to regulation by the FDA. However, it takes between 30–50 months for a medical device (BEM devices classify as medical devices) to get through the FDA, as compared with 144 months for a drug to get through the FDA. A direct correlate of this is that BEM device development is significantly less expensive than pharmaceutical development: BEM devices cost ~$100M for high-risk devices, whereas drug development can require on the order of ~$1B (although the exact cost is uncertain and fluctuates).
Frontiers
There are several frontiers of bioelectronic medicine: technological, scientific, and social/political. Technologically, devices to record and stimulate the nervous system need to be able to record and stimulate at smaller spatial scales and to operate wirelessly (without a wire running from the implanted device, out of the skin, and to a power source). Additionally, neural recordings will be fused with sensors under development that focus on biological signals such as the activity within individual cells. In terms of basic science, researchers need a better understanding and more detailed atlas of the peripheral nervous system and are additionally working to characterize other nervous system reflexes within the body (“reflex” in the sense we described above). Finally, on the social/political front: although public forum discussion of BEM and BMIs is rather small right now, it’s likely that conversation picks up over the next ~5 years as ambitious interface companies like Neuralink, Kernel, and Paradromics hopefully bring progress to bear; almost certainly, the public conversation will focus on privacy and ethics.
Market size: understanding the prospects
To understand the massive market for therapeutic devices that interface with the nervous system, we’ll list six example opportunities and their respective market sizes:
- Spinal Cord Injury – 280,000 patients in the US as of 2016, with a $2.3B global market as of 2017.
- Alzheimer’s Disease – 5.7M patients in the US, with a $1.7B market in North America as of 2017.
- Amyotrophic Lateral Sclerosis – 30K patients in the US, $16M market in the US as of 2018.
- Stroke – 795,000 strokes per year in the US (144,000 deaths per year caused by strokes); $6.5B market in the US as of 2016.
- Rheumatoid Arthritis – 1.5M patients in the US; $11.5B market in the US as of 2016.
- Mental Health/Substance Abuse – 43.8M adults in the US experience mental illness annually; based on data through 2013, the US mental health and substance abuse services industry was expected to produce $53.2B in revenues in 2017.
Expanding beyond these specific use-cases, any new class of treatment and diagnostic methodology like bioelectronic medicine is likely to have effects that trickle down and impact a large portion of the $3.3 trillion dollar US healthcare industry.
In Conclusion
In this piece, we’ve introduced the concept of bioelectronic medicine and brain-machine interfaces: providing therapeutic value to patients by interfacing with their nervous system instead of their bloodstream. We see huge opportunity in this space, driven by novel interfaces to the nervous system and new disease treatment paradigms. As we saw with Ian Burkhart, bioelectronic medicine and brain-machine interfaces are domains with enormous value to add, but whose development will be intricate and require careful ethical considerations. Stay tuned as we dive in-depth to understand the science, technology, and business of bioelectronic medicine.
Special thanks to Ian Burkhart for sharing with us his story and insights. Through the Ian Burkhart Foundation, he works to improve the lives of individuals with spinal cord injuries.
Disclaimer: We actively write about the themes in which we invest: virtual reality, augmented reality, artificial intelligence, and robotics. From time to time, we will write about companies that are in our portfolio. Content on this site including opinions on specific themes in technology, market estimates, and estimates and commentary regarding publicly traded or private companies is not intended for use in making investment decisions. We hold no obligation to update any of our projections. We express no warranties about any estimates or opinions we make.
